Summary of Practice Guidlines
New Relief: Updates in the Treatment of Endometriosis Pain
Endometriosis-related pain can take many forms, including chronic pelvic pain that is cyclical or non-cyclical, dyspareunia, dysuria, and dyschezia. These collectively can have lasting, negative consequences on patient quality of life (QoL).
Endometriosis: Subtypes, Symptoms, and Patient Impact
Endometriosis is an estrogen-dependent gynecologic disease with an elusive mechanistic etiology, resulting in the outgrowth of endometrial tissue beyond the uterus onto other organs such as the fallopian tubes, ovaries, bladder, and more.
Endometriosis: Updates to the Diagnostic Workflow
Historically European Society of Human Reproduction and Embryology guidelines recommended laparoscopy with histology to provide a confirmatory diagnosis of endometriosis, however negative histology did not necessarily…
Interview with Kathryn Garren Inan, WHNP-BC
Kathryn Garren Inan, WHNP-BC
Kathryn Garren Inan, WHNP-BC is the Lead Nurse Practitioner at Ideal Gynecology in Atlanta, GA and is a paid AbbVie consultant. She has a Master of Science in Nursing – Women’s Health Nurse Practitioner from Emory University – Nell Hodgson Woodruff School of Nursing and a Bachelor of Science in Nursing from Anderson University.
Kathryn Garren Inan, WHNP-BC talks Pelvic Pain
“Listening is the first tool that all providers should use with our patients.
Patients will often tell me that I’m the first provider that they’ve ever had to actually listen to them and hear them out.”
Sponsored By: AbbVie US Medical Affairs
Pelvic Pain News
Let’s Get Physical! Musculoskeletal Pelvic Pain
Pelvic floor muscle dysfunction is associated with pelvic pain, physical disability, and sexual dysfunction. Prevalence estimates of musculoskeletal dysfunction in various pelvic pain conditions, including endometriosis, vulvodynia, and painful bladder syndrome, range from 21% to 80%.
Managing Chronic Pelvic Pain
An article in Anaesthesia, the journal of the Association of Anaesthetists, covered the prevalence, challenges to sufferers, and difficulties of managing chronic pelvic pain (CPP). Although CPP is common, there has been little research on how best to manage it. One of the difficulties is the variety of symptoms coming from a variety of organs in a woman’s pelvis.
All About Pelvic Pain
There are a lot of different causes of pelvic pain, and there’s never just one cause of pelvic pain. Many times there is more than one or two, three, or four causes in someone who presents with pelvic pain. And it expands all organ systems. It goes from vascular, urological, gynecological, musculoskeletal, neurological, and dermatological. So, it becomes really difficult to assess someone…
Patient FAQs
Patient Resources
Know Iron Deficiency (ID) in Heart Failure
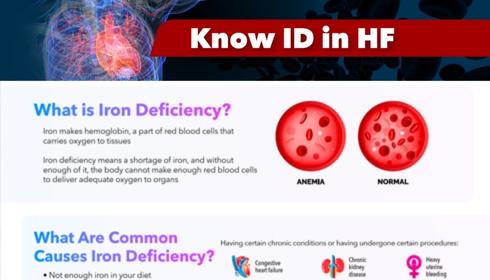
KNOW IRON DEFICIENCY (ID) IN HEART FAILURE What is Iron Deficiency? Iron makes hemoglobin, a part of red blood cells that carries oxygen to tissues
How Is Iron Deficiency Diagnosed And Treated?
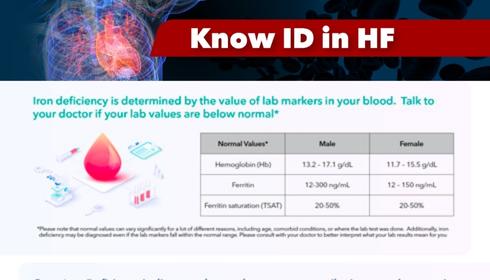
HOW IS IRON DEFICIENCY DIAGNOSED AND TREATED? Iron deficiency is determined by the value of lab markers in your blood. Talk to your doctor if
Iron is an essential element required to produce red blood cells that oxygenate the body and is also an important component of the immune system. Iron is not produced by the human body, it can only be acquired through food intake; it is estimated that only about 20% of the iron consumed from diet can be absorbed, stored and used by the body. In the human body, iron is found in two forms – circulating and stored. Circulating iron is usually more plentiful and is expended first; it exists in the form of hemoglobin (the protein inside red blood cells) and carries oxygen to the organs and tissues of the body. Stored iron, found primarily in the liver, spleen and bone marrow, is in the form of ferritin and hemosiderin and is used when the circulating iron is depleted.
References:
1. University of California San Francisco, “Hemoglobin and Functions of Iron.” Available at https://www.ucsfhealth.org/education/hemoglobin-and-functions-of-iron, accessed September 13, 2021.
2. University of Rochester Medical Center, “What Are Red Blood Cells?” Available at https://www.urmc.rochester.edu/encyclopedia/content.aspx?ContentID=34&ContentTypeID=160, accessed September 15, 2021.
3. Saito, Hiroshi. “Metabolism of Iron Stores.” Nagoya Journal of Medical Science 76.3-4 (2014): 235-254.
Sex differences exist in the amount of iron needed for healthy body function. Adult men need at least 13.0 g/dL of hemoglobin (circulating iron) and adult women need at least 12.0 g/dL, while 800-1000 ng/L of ferritin (stored iron) is a normal level for men, but just 300-500 ng/L is considered normal for women. These differences exist primarily because of body size (men tend to be bigger and thus able to store more iron) and because blood loss during menses often results in women having lower levels.
References:
1. Murphy, William G. “The Sex Difference in Haemoglobin Levels in Adults – Mechanisms, Causes, and Consequences.” Blood Reviews 28.2 (2014): 41-47.
2. Chopra, Vijay K., and Anker, Stefan D. “Anaemia, Iron Deficiency and Heart Failure in 2020: Facts and Numbers.” ESC Heart Failure 7 (2020): 2007-2011.
References:
1. University of California San Francisco, “Hemoglobin and Functions of Iron.” Available at https://www.ucsfhealth.org/education/hemoglobin-and-functions-of-iron, accessed September 13, 2021.
2. University of Rochester Medical Center, “What Are Red Blood Cells?” Available at https://www.urmc.rochester.edu/encyclopedia/content.aspx?ContentID=34&ContentTypeID=160, accessed September 15, 2021.
3. Saito, Hiroshi. “Metabolism of Iron Stores.” Nagoya Journal of Medical Science 76.3-4 (2014): 235-254.
Weakness, fatigue, pale skin, headaches, and dizziness are the most common symptoms of ID. Less common symptoms like restless legs syndrome and pica – the abnormal craving to eat substances like ice, dirt, or paint – may be present in more severe cases of ID, such as ID anemia. Since iron stores are depleted gradually over weeks or months, these symptoms also develop gradually and may be difficult to recognize due to their slow onset. Because many of the symptoms of ID are also signs of other conditions, it is important that patients see their doctor to confirm a diagnosis through blood tests. This is even more important for patients with heart failure (HF), because signs of worsening HF may be mistaken for ID – finding the underlying cause is essential for appropriate treatment.
References:
1. Borgna-Pignatti, Caterina, and Sara Zanella. “Pica as a manifestation of iron deficiency.” Expert review of hematology 9.11 (2016): 1075-1080.
2. Patterson, Amanda J., et al. “Iron deficiency, general health and fatigue: results from the Australian Longitudinal Study on Women’s Health.” Quality of Life Research 9.5 (2000): 491-497.
3. https://www.mayoclinic.org/diseases-conditions/iron-deficiency-anemia/symptoms-causes/syc-20355034
4. van der Wal, Haye H., et al. “Iron deficiency in worsening heart failure is associated with reduced estimated protein intake, fluid retention, inflammation, and antiplatelet use.” European Heart Journal 40.44 (2019): 3616-3625.
When hemoglobin levels are low it indicates that the circulating iron found in red blood cells has been exhausted and the extra iron stored as ferritin in the liver, spleen and bone marrow is being expended. The resulting diagnosis of ID, if not treated, can lead to ID anemia which is more severe.
Both ID and ID anemia are associated with poor outcomes such as reduced quality of life, more frequent hospitalization, worse symptoms, and increased mortality in patients with HF. Guideline from a major professional organization support supplementing iron in patients with HF diagnosed with ID, even if levels aren’t low enough to diagnose ID anemia.
References:
1. McDonagh, Theresa, et al. “Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice.” European Journal of Heart Failure 20.12 (2018): 1664-1672.
2. Anand, Inder S., and Gupta, Pankaj. “Anemia and Iron Deficiency in Heart Failure.” Circulation 138.1 (2018): 80-98.
3. European Heart Journal, “2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure.” Available at https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehab368/6358045, accessed September 13, 2021.
Iron level markers should be taken in the initial diagnostic workup for all patients newly diagnosed with HF, patients with symptomatic chronic HF, and for all other patients with HF at least once a year. These blood tests check the patient’s levels of hemoglobin, serum ferritin and transferrin saturation (TSAT). TSAT is an important marker in assessing iron levels – it is a measure of the ratio of serum iron to total iron-binding capacity. Even if ferritin levels are within normal range, the body cannot efficiently use that iron if TSAT is low. According to guidelines from professional organizations, ID is diagnosed in patients with HF when serum ferritin is less than 100ng/mL, or 100-299ng/ml if transferrin saturation (TSAT) is under 20%.
References:
1. Lam, Carolyn S.P., et al. “Iron Deficiency in Chronic Heart Failure: Case-Based Practical Guidance.” ESC Heart Failure 5.5 (2018): 764-771.
2. Elsayed, M.E., et al. “Transferrin Saturation: A Body Iron Biomarker.” Advances in Clinical Chemistry, Elsevier 2016, 71-97.
3. European Heart Journal, “2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure.” Available at https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehab368/6358045, accessed September 13, 2021.
4. Yancy, Clyde W., et al. “2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association.
Both ID and ID anemia are associated with poor outcomes such as reduced quality of life, more frequent hospitalization, worse symptoms, and increased mortality in patients with HF. Guideline from a major professional organization support supplementing iron in patients with HF diagnosed with ID, even if levels aren’t low enough to diagnose ID anemia.
References:
1. McDonagh, Theresa, et al. “Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice.” European Journal of Heart Failure 20.12 (2018): 1664-1672.
2. Anand, Inder S., and Gupta, Pankaj. “Anemia and Iron Deficiency in Heart Failure.” Circulation 138.1 (2018): 80-98.
3. European Heart Journal, “2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure.” Available at https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehab368/6358045, accessed September 13, 2021.
The mechanisms of ID in HF have been studied extensively, with trials focusing on methods to safely increase iron levels with a goal of improving patient quality of life and reducing HF-related hospitalization and mortality.
Several of these studies reported that oral iron supplementation in the form of an oral iron (ferrous iron tablet) did not satisfactorily increase hemoglobin levels and had unwanted gastrointestinal (GI) side effects. Also, studies have shown that no improvement in exercise capacity or iron levels after oral iron supplementation.
On the other hand, numerous clinical trials showed that patients who were administered intravenous (IV) methods of iron delivery to treat ID in patients with HF had fewer rehospitalizations, improved exercise capacity and better quality of life. To date, IV ferric carboxymaltose is the preferred method of iron repletion in patients with HF, as evidenced by randomized clinical trials.
References:
1. von Haehling, Stephan, et al. “Iron Deficiency in Heart Failure: An Overview.” JACC: Heart Failure 7.1 (2019): 36-46.
2. Lewis, Gregory D., et al. “Oral iron therapy for heart failure with reduced ejection fraction: design and rationale for oral iron repletion effects on oxygen uptake in heart failure.” Circulation: Heart Failure9.5 (2016): e000345.
3. Jankowska, Ewa A., et al. “The effect of intravenous ferric carboxymaltose on health-related quality of life in iron-deficient patients with acute heart failure: the results of the AFFIRM-AHF study.” European Heart Journal 42.31 (2021): 3011-3020.
4. Anand, Inder S., and Gupta, Pankaj. “Anemia and Iron Deficiency in Heart Failure.” Circulation 138.1 (2018): 80-98.
References:
1. Lam, Carolyn S.P., et al. “Iron Deficiency in Chronic Heart Failure: Case-Based Practical Guidance.” ESC Heart Failure 5.5 (2018): 764-771.
2. Elsayed, M.E., et al. “Transferrin Saturation: A Body Iron Biomarker.” Advances in Clinical Chemistry, Elsevier 2016, 71-97.
3. European Heart Journal, “2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure.” Available at https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehab368/6358045, accessed September 13, 2021.
4. Yancy, Clyde W., et al. “2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association.
People with ID are encouraged to introduce more iron-rich foods into their diets, but patients who also have HF should take extra precautions when modifying their eating habits because some iron-containing foods can also have adverse cardiovascular effects. For e.g., red meat is known to contain high levels of iron, but is also linked to high cholesterol, atherosclerosis, and other cardiovascular complications.
Patients with HF that want to safely introduce more iron-containing foods may consider heme (meat) sources other than red meat such as chicken and seafood, or nonheme (nonmeat) foods such as green leafy vegetables, dried dates and prunes, or beans and legumes. Even though these foods contain less iron than red meat, they are not associated with negative cardiovascular problems that could complicate or worsen HF. Ingesting vitamin C-containing foods like citrus fruits with meals helps the body absorb both heme and nonheme iron. Dairy, coffee and tea should be consumed between meals, as calcium and polyphenols may inhibit iron absorption.
One important concern for patients with HF when considering a diet modification is their electrolyte level and renal function. Both should be carefully monitored, as diuretics common in HF treatment can affect both. Hypochloremia (low level of chloride in the body) is the result of electrolyte imbalances and is associated with worse outcomes for patients with HF. For this reason, patients must consult their doctor and may work with a nutritionist to develop a diet that considers the entire scope of their health condition.
References:
1. Cui, Kun, et al. “Association between intake of red and processed meat and the risk of heart failure: a meta-analysis.” BMC Public Health.
2. de Oliveira Otto, Marcia C., et al. “Dietary Intakes of Zinc and Heme Iron from Red Meat, but Not from Other Sources, Are Associated with Greater Risk of Metabolic Syndrome and Cardiovascular Disease.” The Journal of Nutrition 142.3 (2012): 526-533.
3.American Red Cross. “Iron Rich Foods.” Available at https://www.redcrossblood.org/donate-blood/blood-donation-process/before-during-after/iron-blood-donation/iron-rich-foods.html, accessed September 15, 2021.
4. Medicine Net. “Iron and Iron Deficiency Symptoms.” Available at https://www.medicinenet.com/iron_and_iron_deficiency/article.htm, accessed September 15, 2021.
5. Ter Maaten, Jozine M., et al. “Hypochloremia, diuretic resistance, and outcome in patients with acute heart failure.” Circulation: Heart Failure 9.8 (2016): e003109.
6. Cuthbert, Joseph J., et al. “Hypochloraemia in Patients with Heart Failure: Causes and Consequences.” Cardiol Ther 9 (2020): 333-347.